Naturopathic Board Proposes Fictitious Name Permit Program for Consumer Protection
September 29, 2025 | Respiratory Care Board of California, Boards and Commissions, Executive, California
This article was created by AI summarizing key points discussed. AI makes mistakes, so for full details and context, please refer to the video of the full meeting. Please report any errors so we can fix them. Report an error »
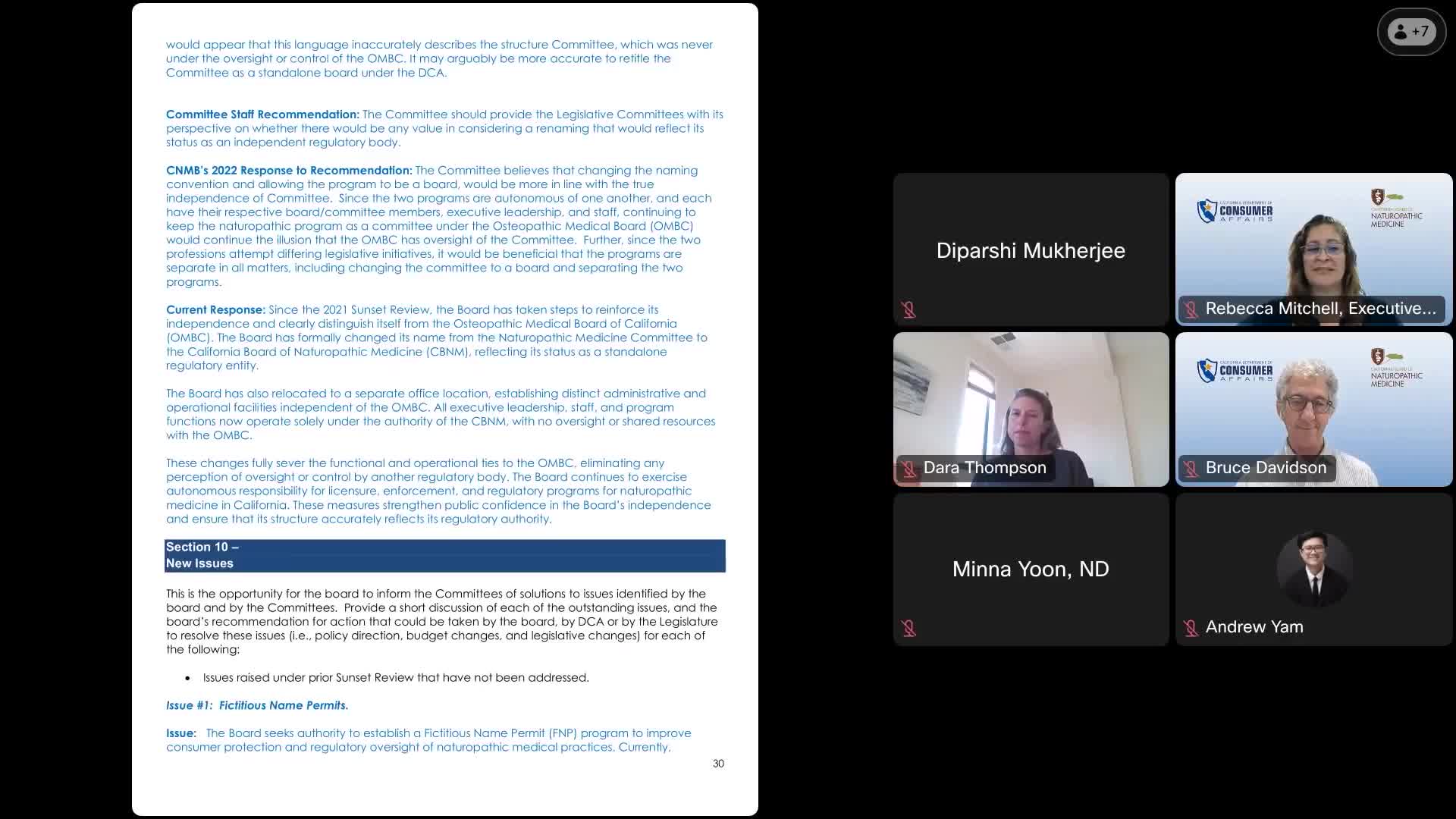
The California Board of Naturopathic Medicine convened on September 29, 2025, to discuss key regulatory updates aimed at enhancing consumer protection and oversight within the naturopathic medical field. The primary focus of the meeting was the proposal to establish a fictitious name permit (FNP) program for naturopathic practices.
The board emphasized the need for this program to improve transparency and accountability in the industry. Currently, consumers often struggle to identify the licensed practitioners behind business names, complicating the process of filing complaints or investigations. The proposed FNP program would require naturopathic licensees to register and disclose ownership of any practice operating under a name other than their own. This aligns with existing practices at the California Medical Board and the Osteopathic Medical Board, which already mandate similar requirements.
The implementation of the FNP program is expected to enhance enforcement capabilities by linking business names directly to licensed naturopathic doctors. This would help address issues where non-licensed practitioners use misleading names that include "naturopathic medicine." The board noted that having a structured naming system would facilitate easier tracking of enforcement issues, allowing for more efficient responses to consumer complaints.
During the meeting, board members discussed the logistics of the FNP program, including the establishment of an application fee to cover processing costs and a renewal fee to ensure fiscal neutrality. Questions were raised regarding the relationship between the fictitious name permits and existing business registrations with the Secretary of State. It was clarified that while businesses must register their names with the Secretary of State, they would also need to obtain a separate permit from the board if they operate under a fictitious name.
The meeting also touched on the potential for the board to publish ownership information on its website, although it was noted that this information would primarily be kept internally. The discussion highlighted the complexities of corporate structures in the naturopathic field, where a corporation may operate under a different name than its registered corporate name.
In addition to the FNP program, a board member raised a question regarding the modification of continuing education requirements, indicating ongoing efforts to adapt regulatory frameworks to better serve practitioners and consumers alike.
Overall, the meeting underscored the board's commitment to improving regulatory oversight and consumer protection in the naturopathic medicine sector, with the FNP program being a significant step forward in achieving these goals. Further discussions and actions are expected as the board moves forward with the proposal.
The board emphasized the need for this program to improve transparency and accountability in the industry. Currently, consumers often struggle to identify the licensed practitioners behind business names, complicating the process of filing complaints or investigations. The proposed FNP program would require naturopathic licensees to register and disclose ownership of any practice operating under a name other than their own. This aligns with existing practices at the California Medical Board and the Osteopathic Medical Board, which already mandate similar requirements.
The implementation of the FNP program is expected to enhance enforcement capabilities by linking business names directly to licensed naturopathic doctors. This would help address issues where non-licensed practitioners use misleading names that include "naturopathic medicine." The board noted that having a structured naming system would facilitate easier tracking of enforcement issues, allowing for more efficient responses to consumer complaints.
During the meeting, board members discussed the logistics of the FNP program, including the establishment of an application fee to cover processing costs and a renewal fee to ensure fiscal neutrality. Questions were raised regarding the relationship between the fictitious name permits and existing business registrations with the Secretary of State. It was clarified that while businesses must register their names with the Secretary of State, they would also need to obtain a separate permit from the board if they operate under a fictitious name.
The meeting also touched on the potential for the board to publish ownership information on its website, although it was noted that this information would primarily be kept internally. The discussion highlighted the complexities of corporate structures in the naturopathic field, where a corporation may operate under a different name than its registered corporate name.
In addition to the FNP program, a board member raised a question regarding the modification of continuing education requirements, indicating ongoing efforts to adapt regulatory frameworks to better serve practitioners and consumers alike.
Overall, the meeting underscored the board's commitment to improving regulatory oversight and consumer protection in the naturopathic medicine sector, with the FNP program being a significant step forward in achieving these goals. Further discussions and actions are expected as the board moves forward with the proposal.
View full meeting
This article is based on a recent meeting—watch the full video and explore the complete transcript for deeper insights into the discussion.
View full meeting
