Article not found
This article is no longer available. But don't worry—we've gathered other articles that discuss the same topic.
Oversight Committee adopts resolution honoring Chief Scientific Officer Michelle LeBeau

University advisory committee presents research portfolio metrics; UTRGV TREC highlighted for regional development

CEO reports favorable legislative outcomes for CPRIT, staffing additions and capital budget for grant-management platform
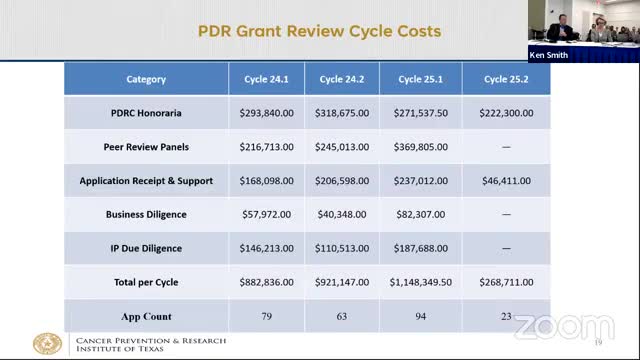
Oversight Committee renews GDIT grant-management contract and authorizes up to $350,000 for Innovation conference venue
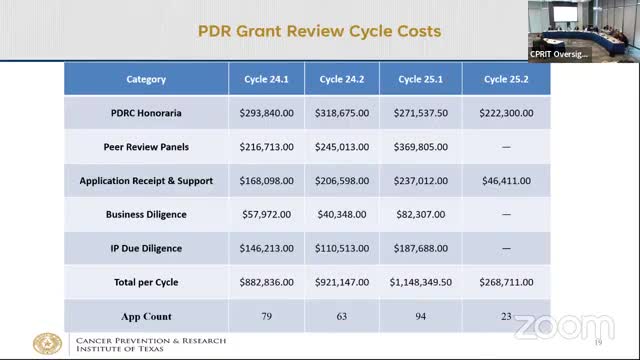
Oversight Committee authorizes up to $300 million bond issuance for FY2026 financing

Internal audit reports on post-award monitoring, non-grant expenditures and purchase-card process presented and approved

Oversight Committee approves eight product-development supplemental grants; authorizes advanced payments for awardees

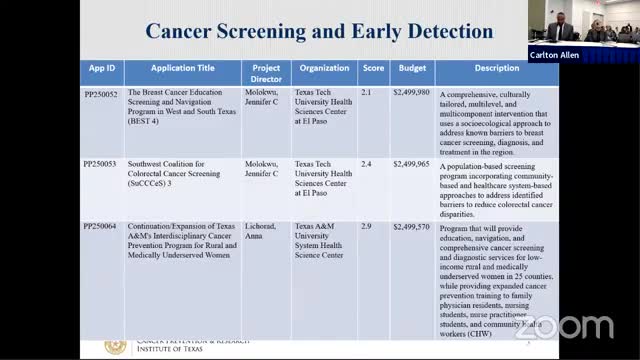
Oversight Committee approves six prevention grants covering screening, HPV vaccination and rural outreach totaling $4.97 million

